> At a Glance
> – Women 30-65 can now self-swab for HPV every five years instead of a Pap smear
> – Insurers must cover the test by January 1, 2027
> – 4,000+ U.S. deaths from cervical cancer occur yearly
> – Why it matters: A quarter of eligible women are behind on screening; the easier option could close that gap
A new federal rule lets millions skip the clinic couch and swab themselves at home-or in the office-for cervical-cancer screening. The move targets the 25 % of women who are overdue for testing and rising cancer rates among thirty-somethings.
How the Self-Swab Works
Insert a slim plastic wand, twist, and mail the sample. Lab analysis checks for high-risk HPV strains that trigger 90 % of cervical cancers. Accuracy matches clinician-collected swabs, and FDA has already cleared two in-office kits plus the $250 Teal Wand for home use after a telehealth visit.
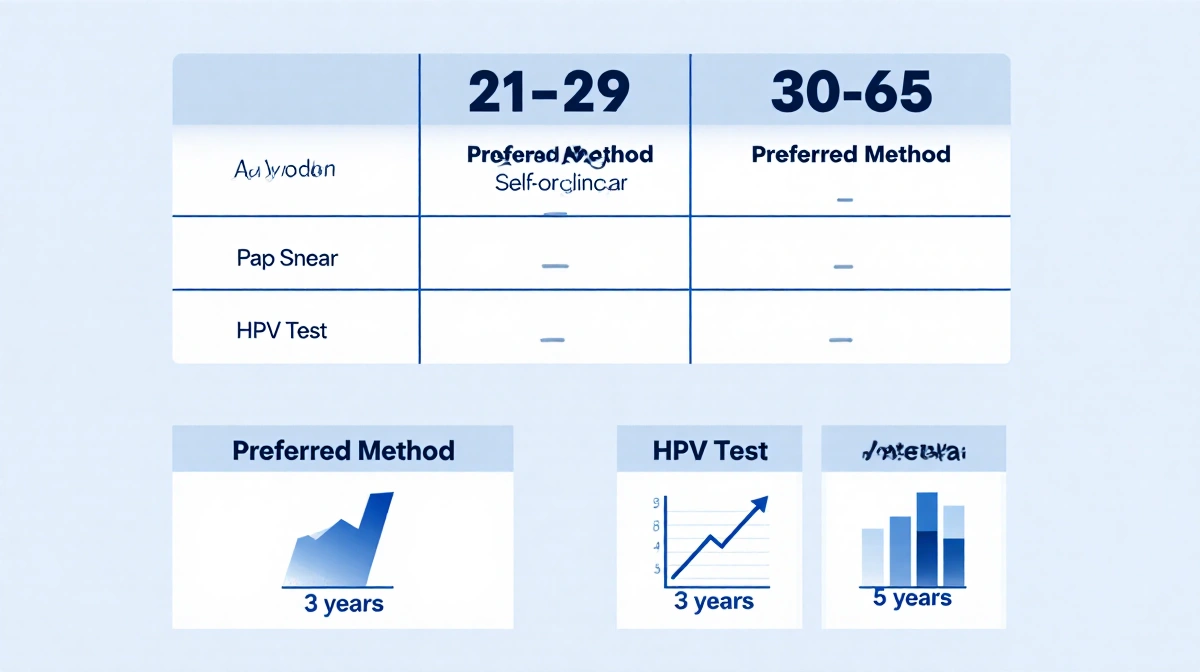
Screening Schedule Under New HRSA Guidelines
| Age Group | Preferred Method | Interval |
|---|---|---|
| 21-29 | Pap smear | 3 years |
| 30-65 | HPV test (self or clinician) | 5 years |
Doctors must still offer Pap smears, but HPV testing becomes the front-line option for women 30 and older.
Insurance Impact
Most private plans are bound by HRSA’s preventive-service list, so they’ll pick up the tab-including any follow-up tests-once the rule takes effect in 2027. Until then, patients may pay out of pocket for at-home kits.
Key Takeaways
- Self-swab HPV tests every five years replace Pap smears for average-risk women 30-65
- Coverage becomes mandatory for insurers on January 1, 2027
- The change could boost screening in rural or clinic-scarce areas
The new option arrives as cervical-cancer cases inch upward among women in their 30s and early 40s, a trend officials blame on missed screenings and lagging HPV vaccination rates.




